MANTA: The Maastricht ANTiarrhythmic drug evAluator
MANTA is a novel educational tool for understanding the basic cellular electrophysiological features of antiarrhythmic drugs (AADs). It enables the user to run cellular simulations under different computational models of cardiac electrophysiology and observe the effect of AADs in various environmental conditions. MANTA is a computational modeling software that employs Myokit, a tool for cardiac cellular electrophysiology simulations developed by Clerx et al. Therefore, Myokit needs to be installed according to the installation guide that can be found on the Myokit website before MANTA can be installed. After installing Myokit, open the Anaconda Prompt from the start menu and type:
pip install manta_maastricht
When the installation of MANTA is finished, MANTA can be started by typing in the Anaconda Prompt:
python -m manta_maastricht
Subsequently, the MANTA user interface shown below will be displayed and MANTA is now ready for use. For more user information, please refer to the Supplementary Materials of our paper in Pharmacol. Res.
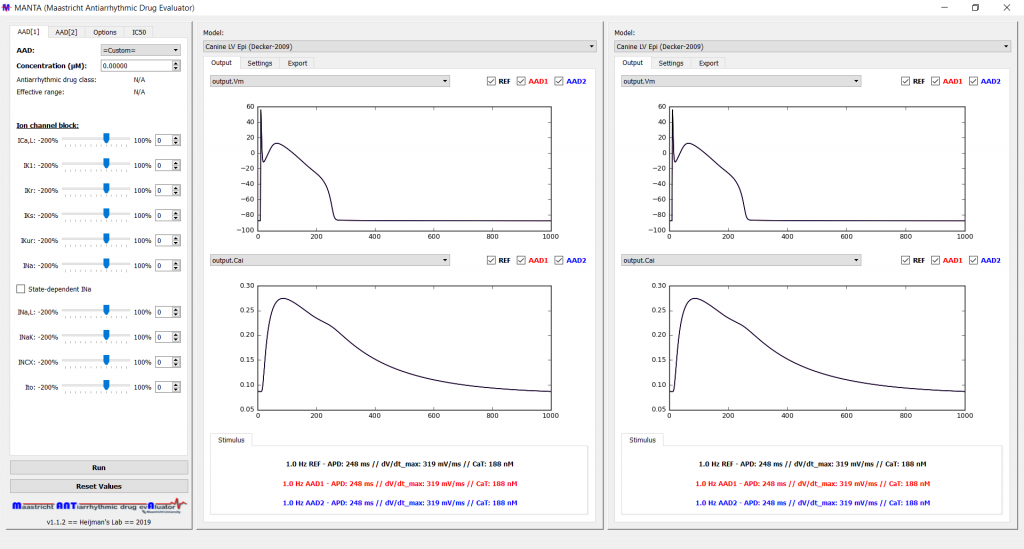
NOTE: Current version of MANTA (v1.1.2) was built under Microsoft Windows OS, and has not been tested in another OS, including Apple MacOS or Ubuntu Linux.
Reference: Sutanto H, Laudy L, Clerx M, Dobrev D, Crijns HJGM, Heijman J (2019) Maastricht antiarrhythmic drug evaluator (MANTA): a computational tool for better understanding of antiarrhythmic drugs. Pharmacol Res, Manuscript accepted on September 3, 2019
Impact of subcellular distribution of calcium-handling proteins in atrial cardiomyocytes
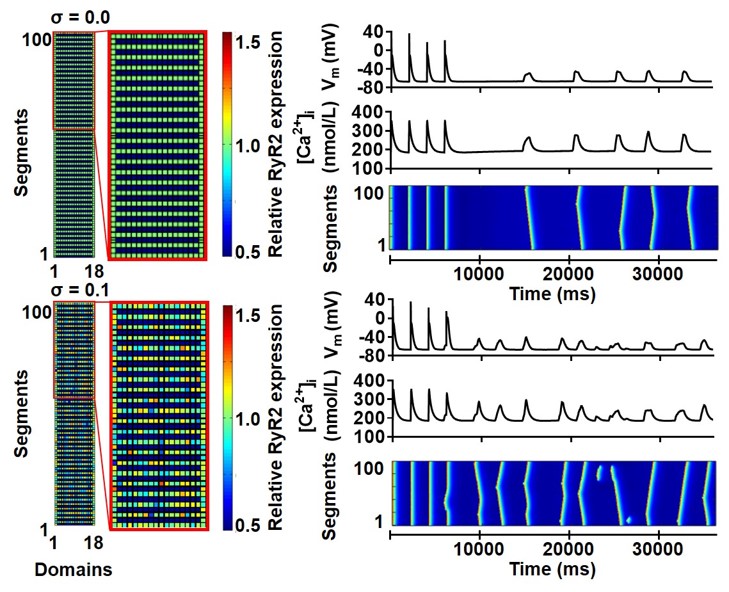
We have recently extended our previous spatial calcium-handling model of the human atrial cardiomyocyte (Voigt, Heijman et al. Circulation 2014) to simulate heterogeneous distributions of ryanodine receptor (RyR) channels and L-type calcium channels (in axial tubules). We employed the perfect control and observability provided by this computer model to overcome experimental challenges in the analysis of the subcellular determinants of cardiomyocyte calcium handling. Our work identifies an increased SCaE incidence for larger heterogeneity in RyR expression (Figure), in which SCaEs preferentially arise from regions of high local RyR expression. Importantly, whole-cell calcium-handling properties are determined by nonlinear interactions between heterogeneities in the properties (expression, phosphorylation) of both L-type calcium channels and RyR, highlighting the need for detailed immunocytochemistry and functional studies to explain differences in whole-cell calcium-handling properties between conditions.
The computer model employed in this study (a combination of C++ and Octave code) can be downloaded using the link below:
Human atrial myocyte model with spatial calcium handling and RyR heterogeneity used in Sutanto et al. Front Physiol.
Reference: Sutanto H, van Sloun B, Schönleitner P, van Zandvoort MAMJ, Antoons G, Heijman J (2018) The Subcellular Distribution of Ryanodine Receptors and L-type Ca2+ Channels Modulates Ca2+-transient Properties and Spontaneous Ca2+-release Events in Atrial Cardiomyocytes. Front Physiol, Manuscript accepted on July 23, 2018